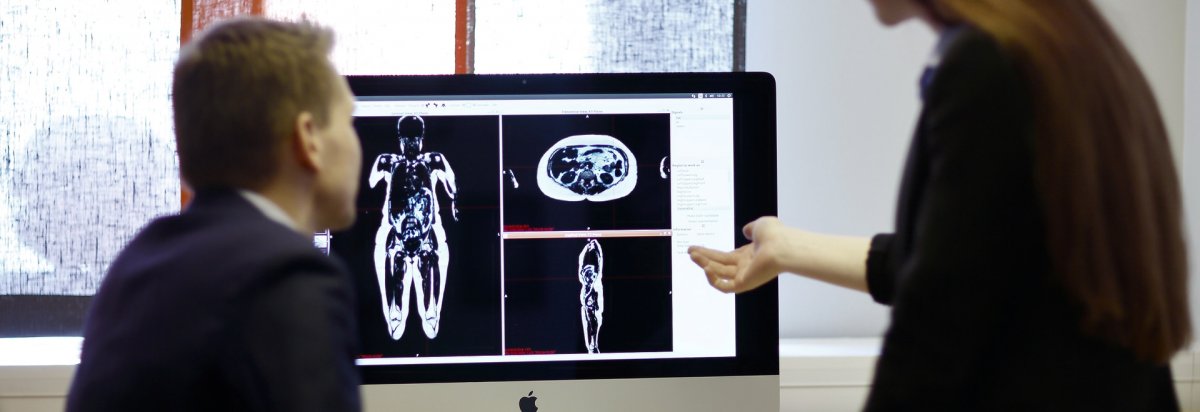
AMRA Medical, a company based in Linköping, Sweden, won FDA clearance to introduce its AMRA Profiler technology for fat and muscle composition analysis.
The product relies on a six minute MRI scan, performed using a compatible scanner, with the imaging data being sent to AMRA’s servers. AMRA, in turn, delivers individualized reports that can be read by physicians and shared with patients.
The AMRA Profiler is the first product able to convert a short MRI into a high quality 3D fat and muscle map, and provide the metabolic status, of the patient’s body. Specifically, the technology gives visual and values-based information on the visceral adipose tissue volume, subcutaneous adipose tissue volume, liver fat fraction, and thigh muscle volume.
The AMRA Profiler does require a compatible MRI scanner, and the company works with providers to make sure the offering is installed so that there’s very little interaction that is required to receive body composition analysis reports for every patient getting the appropriate scan.
"We are delighted with the FDA’s decision. The challenges facing healthcare systems across the world are well-documented. Cost constraints, together with societal issues such as obesity and an aging population, are putting hospitals and private clinics under increasing pressure, in a published statement said Eric Converse, CEO of AMRA Medical. AMRA® Profiler helps address these challenges by providing physicians with the most detailed body composition assessment and imaging available, cost-effectively and with minimal intrusion to the patient. Ultimately this enables clinicians to make more informed treatment decisions about the whole body. This clearance is the next step in our journey of translating the benefits of AMRA into clinical practice and in ultimately contributing to the real world data and real world evidence that are playing an increasing role in health care decisions today."