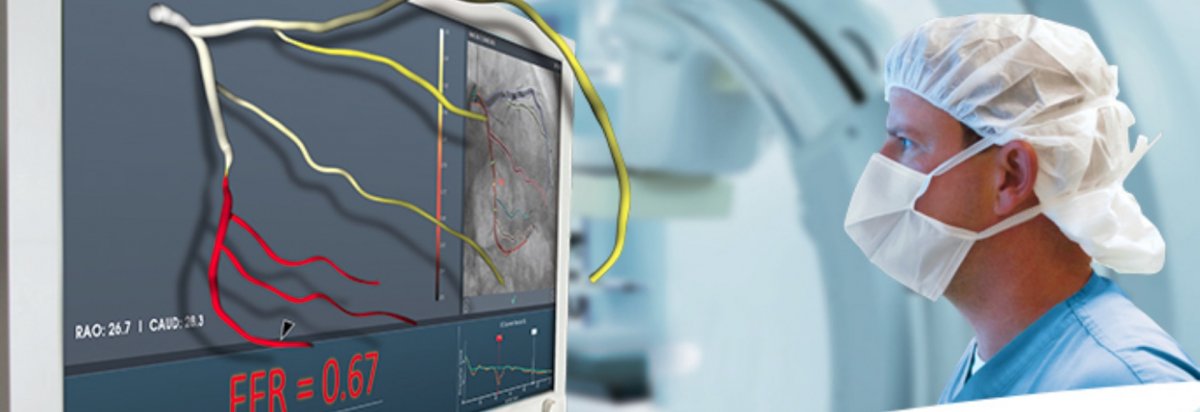
Fractional flow reserve (FFR) is a measurement of the blood pressure before and after a narrowing of an artery. The larger the difference in the pressure across the stenosis, the more need there is to perform an interventional procedure to open up the artery.
Normally, FFR is measured using a special intravascular catheter and this is now quite common. Nevertheless, traditional FFR involves its own procedural risks, takes a good deal of time to perform, and adds substantial costs.
CathWorks, a company with offices in Israel and California, has just won FDA clearance for its FFRangio system, a non-invasive alternative to traditional FFR. The FFRangio uses only X-rays obtained during angiography as its input data, eliminating any need for any additional implements or steps to be taken to obtain what the company claims to be ?substantially equivalent? results.
After performing its calculations, the FFRangio system displays an easy to view coronary network with all the FFR values labeled along each major vessel.
"The FDA clearance of CathWorks FFRangio is a significant milestone for interventional cardiologists and the healthcare system overall,? said Jim Corbett, CEO of CathWorks. ?It is the first non-invasive device of its kind to receive FDA clearance for use during Percutaneous Coronary Intervention (PCI) assessment. The FAST-FFR study was carried out at 10 centers world-wide and evaluated more than 380 patients. The study demonstrated the clinical predictive value across a full range of coronary physiology, including complex lesion assessment in bifurcations and calcified lesions. FAST-FFR also demonstrated that the FFRangio system could perform non-invasive, objective, multi-vessel, physiologic measurements to support PCI decision making."
FFRangio Uses X-Rays to Measure Fractional Flow Reserve, Now Cleared in U.S.
InfoWeb Marketplace